
Communication and sharing promote growth
Joining Hands for Development!

Aluminum alloy is the most widely used non-ferrous metal structural material in industry, especially in scenarios where the thermal conductivity of materials is of great concern, and in situations where efficient heat conduction is required, such as electronic equipment heat dissipation, electric vehicle three-power heat dissipation, and battery energy storage systems. In the fields of heat dissipation and aerospace, it is usually used to manufacture efficient heat transfer equipment such as radiators, heat conduction plates, and electronic components.
Thermal conductivity, also called thermal conductivity, is a parameter index that characterizes the thermal conductivity of materials. It indicates the heat conduction per unit time, unit area, and negative temperature gradient. The unit is W/m·K or W/m·℃. Aluminum alloy is an alloy material composed of aluminum and other metals. Its thermal conductivity is very excellent, and the thermal conductivity coefficient is usually between 140-200W/(m·K). As the metal with the highest content in the earth's crust, aluminum has a relatively low thermal conductivity coefficient. It is favored because of its high height, low density and low price.
1-Thermal conductivity principle of aluminum alloy materials
When there is a temperature difference between adjacent areas of a material, heat will flow from the high-temperature area to the low-temperature area through the contact part, resulting in heat conduction. There are a large number of free electrons in metal materials. Free electrons can move quickly in the metal and can quickly transfer heat. Lattice vibration is another way of metal heat transfer, but it takes a back seat compared to the free electron transfer method.
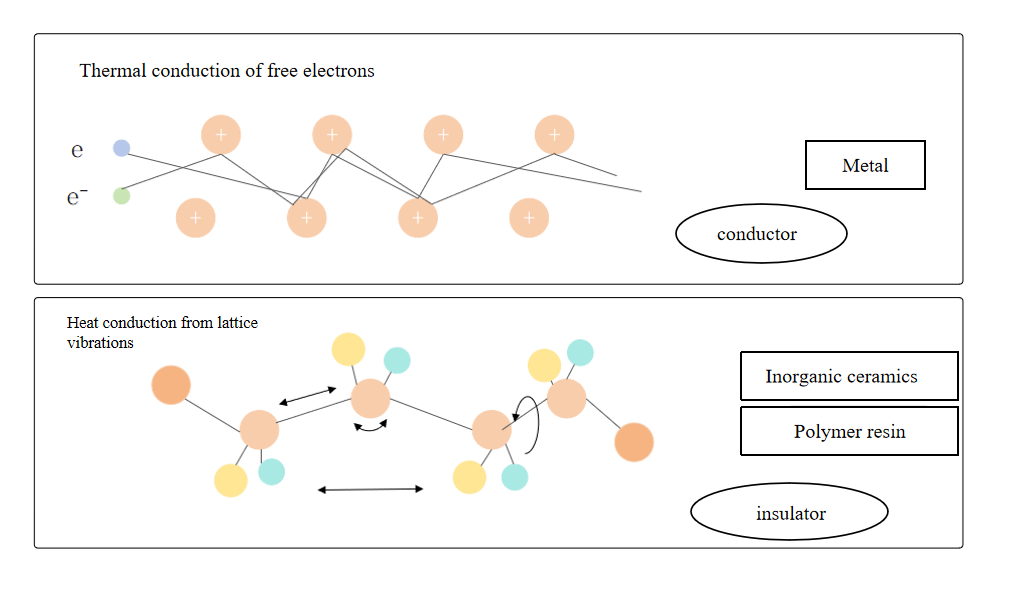
Comparison of heat conduction methods between metals and non-metals
2-Factors affecting the thermal conductivity of aluminum alloys
a.Alloying is one of the main factors affecting thermal conductivity. Alloying elements exist in the form of solid solution atoms, precipitated phases and intermediate phases. These forms will bring crystal defects, such as vacancies, dislocations and lattice distortion. These defects will increase the probability of electron scattering, resulting in a reduction in the number of free electrons, thereby reducing the Thermal conductivity of alloys. Different alloying elements produce different degrees of lattice distortion on the Al matrix, and have different effects on thermal conductivity. This difference is the result of multiple factors such as the valence of alloy elements, atomic volume differences, extranuclear electron arrangement, and solidification reaction type.
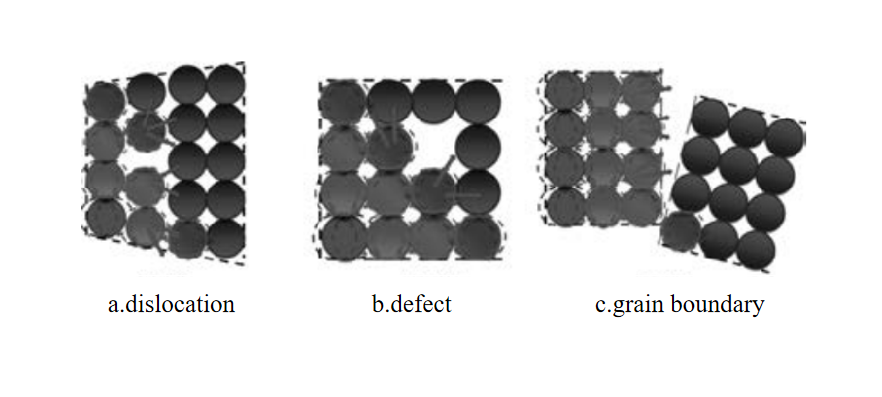
b.Heat treatment is a very important step in the processing of aluminum alloys. By changing the microstructure and phase transformation of aluminum alloys, its thermal conductivity can be significantly affected. Solid solution treatment is to heat the aluminum alloy to a certain temperature to fully dissolve the solute atoms in the matrix, and then quickly cool it to obtain a uniform solid solution. This treatment improves the material's mechanical properties but usually reduces its thermal conductivity. Aging treatment is through appropriate cold deformation and reheating after solid solution treatment, which can optimize the microstructure of the alloy and improve its overall performance. The aging treatment takes into account the mechanical properties and thermal conductivity of the alloy, so that the alloy maintains high strength while also having good thermal conductivity. Annealing improves the microstructure of the alloy by maintaining it at a lower temperature to precipitate and redistribute the second phase in the alloy. Annealing treatment can improve the plasticity and toughness of aluminum alloys, but the effect on thermal conductivity varies depending on the specific situation.
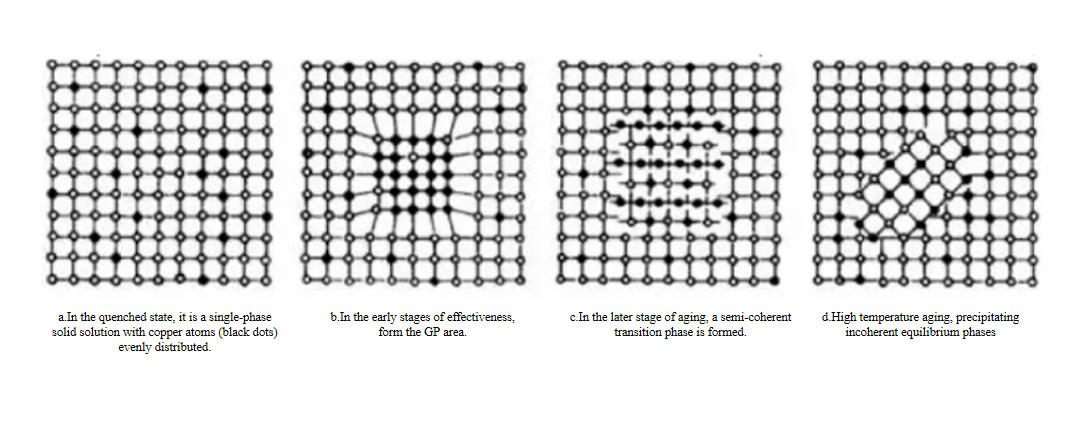
Schematic diagram of crystal structure changes during the aging process of Al-Cu alloy
c.Other factors influence, impurities and second phase particles: Impurities and second phase particles (such as oxides, carbides, etc.) in aluminum alloys can scatter hot carriers (electrons and phonons), thereby reducing thermal conductivity. The higher the impurity content, the coarser the second phase particles and generally the lower the thermal conductivity. The grain size of aluminum alloys also affects thermal conductivity. Generally speaking, the smaller the grain size, the more grain boundaries there are and the lower the thermal conductivity. In addition, the processing method of aluminum alloy (such as rolling, extrusion, forging, etc.) will affect its microstructure and residual stress state, thereby affecting the thermal conductivity. Work hardening and residual stresses reduce thermal conductivity.
In summary, aluminum alloy is an ideal choice for high thermal conductivity materials. Factors such as the type of alloy elements in aluminum alloys and their forms, heat treatment methods, impurities, grain size, and molding methods will all affect the thermal conductivity of aluminum alloy materials. Comprehensive considerations should be taken when designing material composition and process planning.
We will regularly update you on technologies and information related to thermal design and lightweighting, sharing them for your reference.Thank you for your attention to Walmate.